COVID 19
-
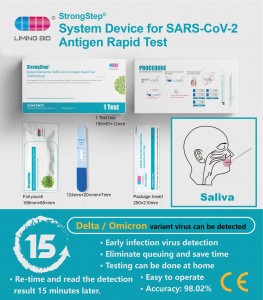
Strongstep ratio fabrica pro Sars, Cov-II antigen celeri test
Ref (L) CCX Specificatio I test / arca Deprehensio principle Immunochromatographic Specimens SalivaIntendebat usum STRADEPRatio fabrica pro Sars-Cov, 2Antigen celeri test Emundorum Immunochromatography Technobgy ad deprehendere Sars-Cov- II nucleocapsid antigen in humana saliva. Hoc test est una tantum usum et animo ad sui temptationis. Est suadetur ut hoc test in VII dies indicium impetu. LT est sustinetur a dinical euismod aestimationem. -

Sars, Cov-II antigen celeri test (nasi)
Ref (D) CC Specificatio I probat / Box; V probat / Box; XX probat / Box Deprehensio principle Immunochromatographic Specimens Anterior nasi swab Intendebat usum Strongstep® Sars, Cov-II antigen celeri Test Paperback Employs Immunochromatography Technology ad deprehendere Sars- Cov-II nucleocapsid antigen in humana anterior nasi Swab Specimen. Hoc testis una tantum usum et in animo pro se testing. Recommanded ut hoc test intra V dies signum impetu. Firmatur per orci perficientur taxationem. -

Sars, Cov-II antigen celeri test (professional usu)
Ref (D) CC Specificatio XXV probat / Box Deprehensio principle Immunochromatographic Specimens Anterior nasi swab Intendebat usum Strongstep® Sars, Cov-II antigen celeri Test Paperback Employs Immunochromatography Technology ad deprehendere Sars- Cov-II nucleocapsid antigen in humana anterior nasi Swab Specimen. Hoc testis una tantum usum et in animo pro se testing. Recommanded ut hoc test intra V dies signum impetu. Firmatur per orci perficientur taxationem. -
1人份抗原卡实物图唾液版1_00_副本-300x216.png)
Sars, Cov-II Antigen celeri test enim saliva
Ref (D) CCXXX Specificatio XX probat / Box Deprehensio principle Immunochromatographic Specimens SalivaIntendebat usum Hoc est celeri Immunochromatographic Assay pro Deprehensio Sars-Cov-II Viris nucleocapsid protein antigen in humana saliva swab collegit ex hominum qui suspected in Covi-XIX in curis provisor intra primos quinque diebus impetu sympants. Et asay adhibetur ut ad auxilium in diagnosis de Covi-XIX. -

Ratio fabrica pro Sars, Cov-II & Influenza A / B Combo Antigen celeri Test
Ref (D) CCXX Specificatio XX probat / Box Deprehensio principle Immunochromatographic Specimens Nasi / Oropharyngeal Swab Intendebat usum Hoc est celeri Immunochromatographic Assay pro Deprehensio Sars-Cov-II Virus nucleocapsid protein antigen in humana nasi / Oropharyngeal Swab ex hominum qui suspicionem Covi-XIX in curis provisor intra primum quinque dies impetu symptoms. Et asay adhibetur ut ad auxilium in diagnosis de Covi-XIX. -

Dual Biosafety ratio fabrica pro Sars, Cov-II antigen celeri test
Ref (L) CCX Specificatio XX probat / Box Deprehensio principle Immunochromatographic Specimens Nasi / Oropharyngeal Swab Intendebat usum Hoc est celeri Immunochromatographic Assay pro Deprehensio Sars-Cov-II Virus nucleocapsid protein antigen in humana nasi / Oropharyngeal Swab ex hominum qui suspicionem Covi-XIX in curis provisor intra primum quinque dies impetu symptoms. Et asay adhibetur ut ad auxilium in diagnosis de Covi-XIX. -

Novel Coronavirus (Sars-Cov- II) Multiplex Real-vicis PCR ornamentum
Ref (DCXC0 Specificatio XCVI probat / Box Deprehensio principle PCR Specimens Nasi / nasopharyngeal swab Intendebat usum Hoc est in animo esse ad consequi qualitative deprehendatur Sars-Cov-II viral RNA extrahitur ex Nasopharyngeal Swabs, Oropharyngeal Swabs, Sputum et Balf ex aegris in consociatione et designatur PCR platforms superius. In ornamentum est in animo usus per officinarum exercitati personas
-

Sars, Cov-II & Influenza A / B multiplex realis-vicis PCR ornamentum
Ref (LI )c Specificatio XCVI probat / Box Deprehensio principle PCR Specimens Nasi / nasopharyngeal swab / Oropharyngeal Swab Intendebat usum Strachsep® Sars, Cov-II & Influenza A / B multiplex realis-vicis PCR ornamentum est in animo et simultaneative deprehensio et differentiation of Sars-COV-II, influenza in Humulte et Influenza B virus et Nasopharynge Aut Oropharyngeal Swab specimens et auto-collected nasi vel Oropharyngeal Swab specimens (colligit in curis occasum cum disciplinam a curis provisor) ab hominibus suspectus respiratoriorum viral infectio consistent cum Covi- XIX a curis provisor.
In ornamentum est in animo usus per officinarum exercitati personas
-

Sars, Cov-II IGM / IGG Antibody celeri Test
Ref (DMDXC Specificatio XX probat / Box Deprehensio principle Immunochromatographic Specimens Omnis sanguis / Serum / Plasma Intendebat usum Hoc est celeri Immuno-Chromatographic Assay ad Simultaneous Deprehensio IGG et IGG antibodies ad Sars, Cov- II virus in humana toto sanguine, serum vel Plasma. In test limited in US ad distribution ad laboratoriosam certified by clia ad praestare princeps complexionem temptationis.
Hoc test non recensuit per FDA.
Negative praecessi non tollit acuti Sars, Cov-II infectio.
Results ab Antibody testis ne solebat egritudo aut excludere acuti Sars, Cov- II infectio.
Positive results potest esse ex praeteritis vel praesens infectio cum non-Sars-Cov-II coronavirus modos, ut coronavirus Hku1, NL63, OC43, aut 229E.







