Nuper, StrongStep® SARS-CoV-2 Antigen Celeri Test (Professional Edition) ex Nanjing Liming Bio-products Co., Ltd. Singapore HSA certificationem, Malaysia (MDA) album commendatum obtinuit, et in UK Department of Health ac Officia humana (DHSC) independenter aestimantur et laudantur.
Ante hoc, StrongStep® SARS-CoV-2 antigenum detectionis ornamentum a Nanjing Liming Bio-products Co, Ltd. successive EU CE certificationem consecutus est, Sinarum Institutionum Nationalium pro Cibus et medicamentis (NIFDC) inspectionis verificationis, Rockefeller intravit. Fundamentum commendatur album, procuratio foederalis Germaniae medicinarum et certificationum medicinalium (BfArM) certificationis, certificationis Guatimalensis, certificationis Indonesianae FDA, Ministerio Italiae Sanitatis certificationis, Philippines FDA certificatione, Singapore HSA certificatione, Aequatoria certificatione, Brasilia (ANVISA) certificatione, Chile certificatione , Argentina certificationis, Dominica certificationis, Guatimalensis certificationis et aliorum certificationum.In statu, Africa Australis, India, QUI EUL, FDA EUA, album Europae album aliaeque certificationes applicationes in progressu sunt.
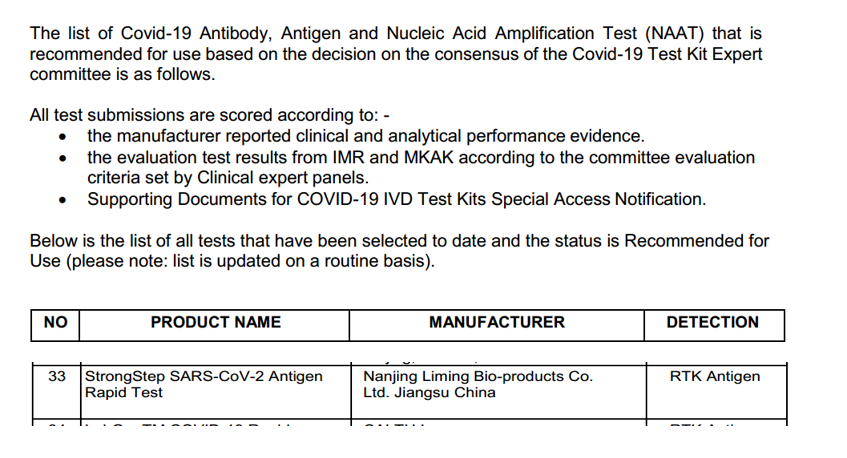
Image source: Commendatur a Ministerio Malaysian Health

Picture: Singapore HSA certificatione
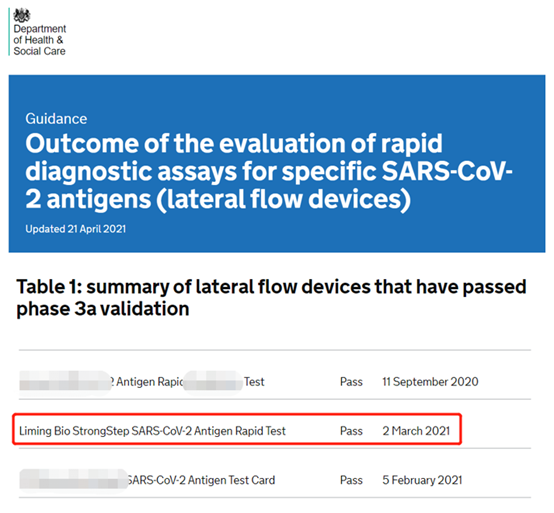
(Image source: DHSC officialis Britanniae Department of Health and Human Services)
Anno 2020, Department Salutis et Humani Muneris in Britannia, stricte comprobabit celeris diagnostica reagentia pro COVID-19 regione ingressa ut satis accurate et certa sint.Sunt 120 producta participationes processus verificationis, quorum solum 19 producta verificationem transierunt.Post sex menses severitatis iteratae verificationis et verificationis, 200 specimina positiva et 1,000 specimina negativa plene probata superiori observantia Nanjing Liming Bio-products Co., Ltd. detectionem celerem pro COVID-19.
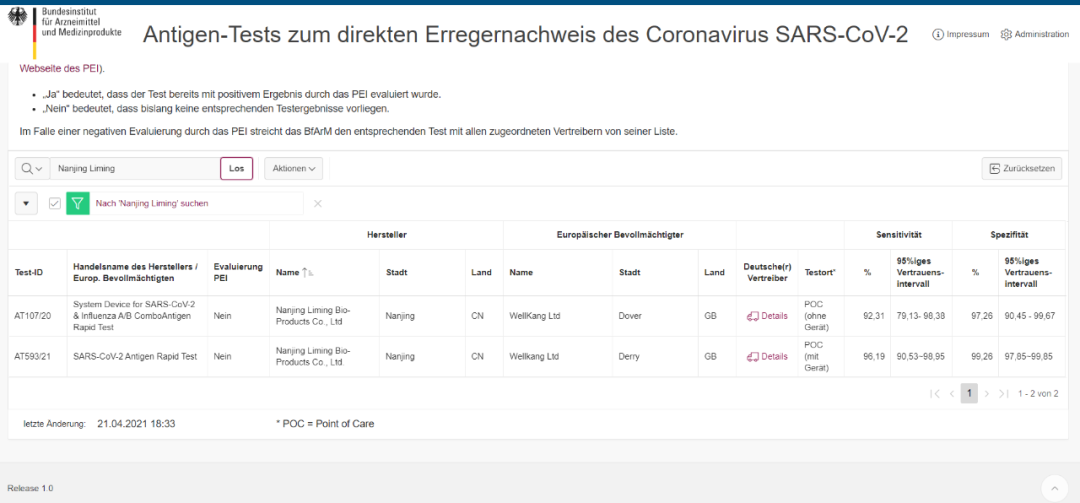
(Image source: German officialis Federal Agency for Medicine and Medical Devices (BfArM) website)
Officialis certificationis Test-ID: AT593/21
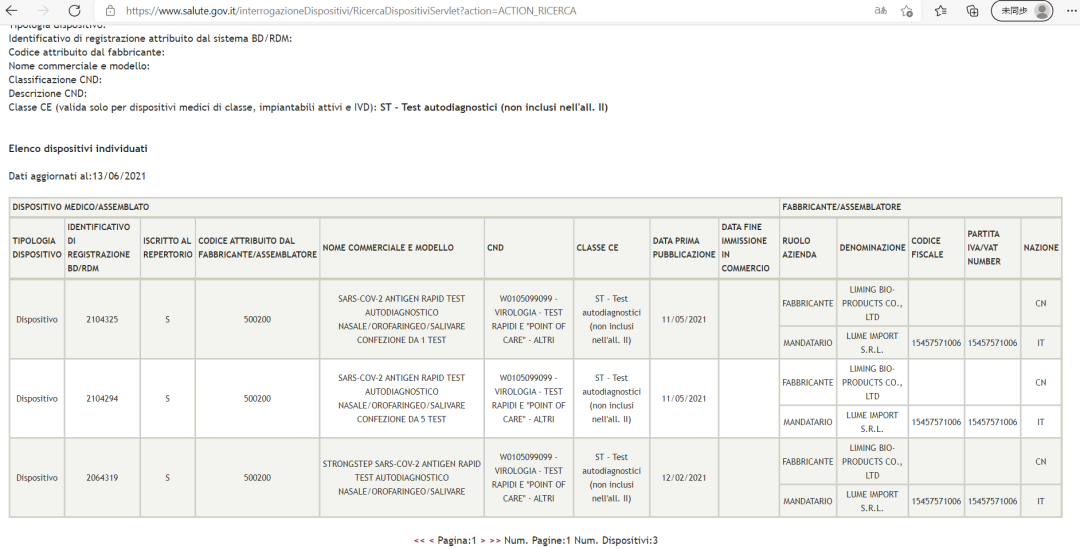
StrongStep® SARS-CoV-2 Antigen Celeri Test versionis auto-testationis (Classical genus sui) probata est a Ministerio Sanitatis Italico
Source: Official website Ministerii Salutis Italiae (Ministero della Salute)

StrongStep® SARS-CoV-2 Antigen Celeri Test laudata et commendata ab usoribus Italicis
Testium antigenum Sars-CoV-2 celere, accuratum, simplex est ad operandum, et apparatum et personas humilis requirit.Aptissima est celeri investigatione suspectorum casuum magnae-scalae novae virus infectio, praesertim ad celeri diagnosis motuum congestorum.Prima linea defensionis pro epidemica potestate adhiberi potest, applicata ad detectionem contagionum antiquarum, ad epidemiam praeventionis et temperantiae subveniendi, et propagationem virium regendi.
Covid-19 erit in longa condicione pestilentia in futuro, et postulatio probationis acriter augebit.Pro diversa applicatione missionum, Nanjing Liming Bio-products Co., Ltd. varias deprehendendi reagentes SARS-CoV-2 processit, "SARS-CoV-2 detectio acidi nucleici + SARS-CoV-2 detectio antigenis + SARS-CoV- 2 anticorpus detectio + SARS-CoV-2/A et B antigenus triplex celerior test + SARS-CoV-2/A et B nuclei acidum triplicem testium + SARS-CoV-2 familiae propriae inspectionis "Solutionis plenae-solutionis necessitatibus occurrit. deprehensionis et deprehensionis ad omnes gradus in foro globali.Comprehensive adiuvant praeventionem et potestatem globalis COVID-19 pestilentiae et praeventionis et moderati morborum respiratorii sicut influentia.
Post tempus: Iun-23-2021







